High pressure freezing, freeze-substitution
Chemical fixation at room temperature takes order of magnitude longer because it takes that long for fixatives to diffuse through the cell. HPF is the choice of fixation when best preservation of ultrastructure is needed (e.g. for structural studies at high resolution and for electron tomography) or when chemical fixatives have to be avoided (e.g. immunolabelling with aldehyde-sensitive antigens/antibodies).
HPF-fixation is followed by freeze-substitution where water in the cells is substituted by organic solvent at -80 - -90° C, and fixatives (such as glutaraldehyde, uranyl acetate or osmiumtetroxide) are introduced. Fixatives in substitution medium diffuse into the cells while all cellular components are immobilized by low temperature, and when substitution medium is warmed up to the temperature that permits chemical cross linking, the fixatives are already dispersed throughout the cells and fixation occurs immediately. Embedding and polymerization are done at low temperatures (-30 - -55° C).
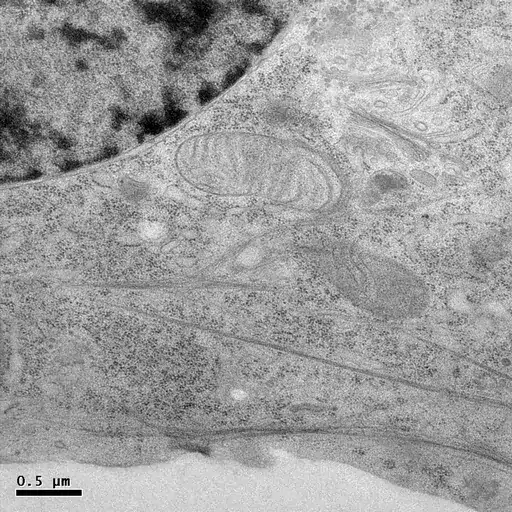
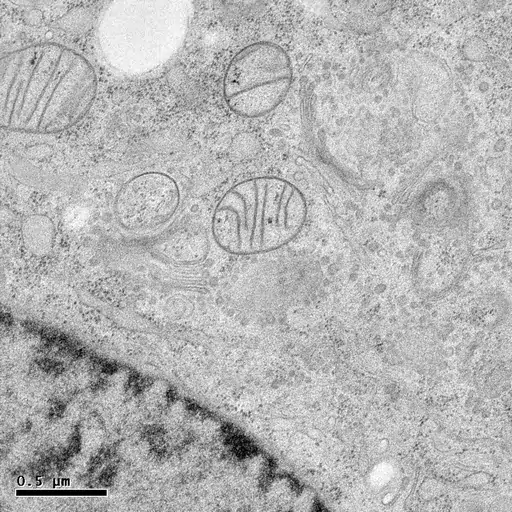
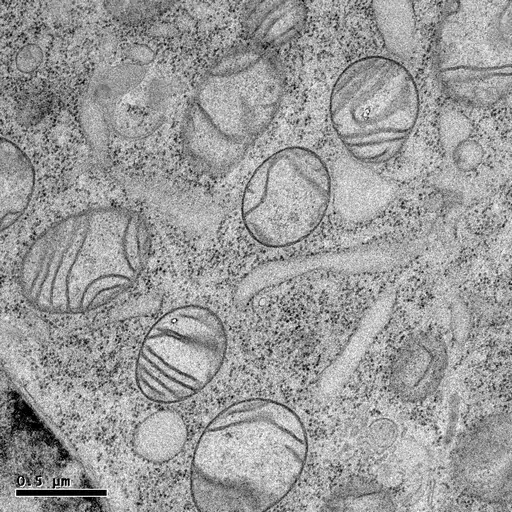
HPF followed by freeze-substitution with 0.5% uranyl acetate in methanol results in excellent preservation of actin-filaments in NRK cells grown on sapphire coverslips (M. Lindman).