Our mission
All our analytical services are available for users from both academia and industry, either as collaborative research projects or commercial analytical services. The experiments can be conducted either by on-site users or as a mail-in service.
What can we offer?
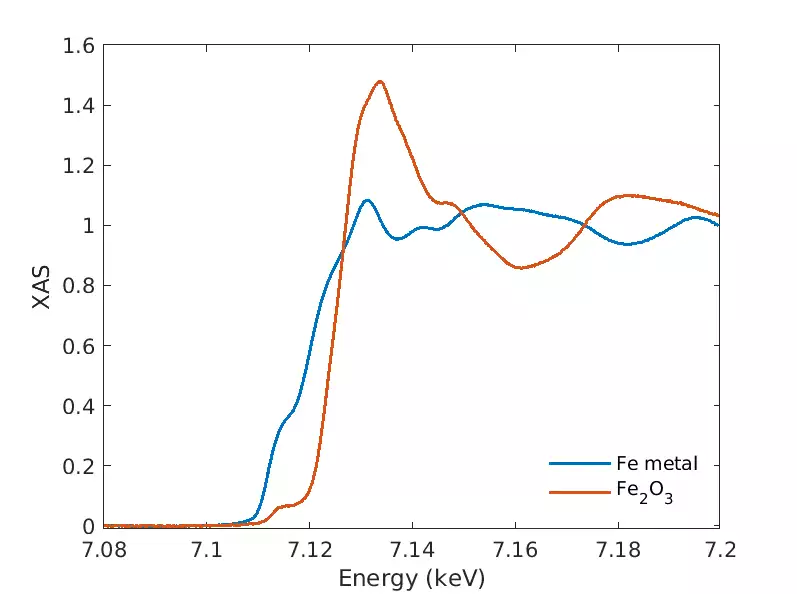
Our x-ray spectroscopy tools give information on the chemical state and atomic near-neighbour coordination of a given element. When tuning the x-ray energy to an inner core electron absorption energy, or measuring the fine structure of an fluorescence spectrum, detailed element-specific chemical and physical information can be obtained.
X-ray absorption spectroscopy (XAS, either by x-ray absorption near edge spectroscopy XANES, and extended x-ray absorption fine structure EXAFS) as well as high-resolution x-ray emission spectroscopy (XES) can be obtained from elements in the periodic table below.
XANES gives information on the oxidation state of an element, its local coordination symmetry and speciation. EXAFS gives information on the near-neighbour atomic shell distances, coordination numbers and local disorder.
XES can give information on the spin and valence state of an element as well as on the ligands bound to a given ion through so-called valence-to-core spectroscopy.
The elements that can be studied at our local laboratory-based instrumentation are limited by the electron binding energies and available monochromator crystals. Currently we limit our research to use of x-rays with photon energies between 5-25 keV*, which covers most elements starting from Ti. In the table those elements that have been proven to be measurable in a routine manner are marked with dark colors. For those that we in principle have capabilities for but have not yet been tested at our facility, are marked with lighter colors. White background around a given element means it is not deemed feasible, either binding energies falling outside our energy range or we still lack a suitable monochromator.
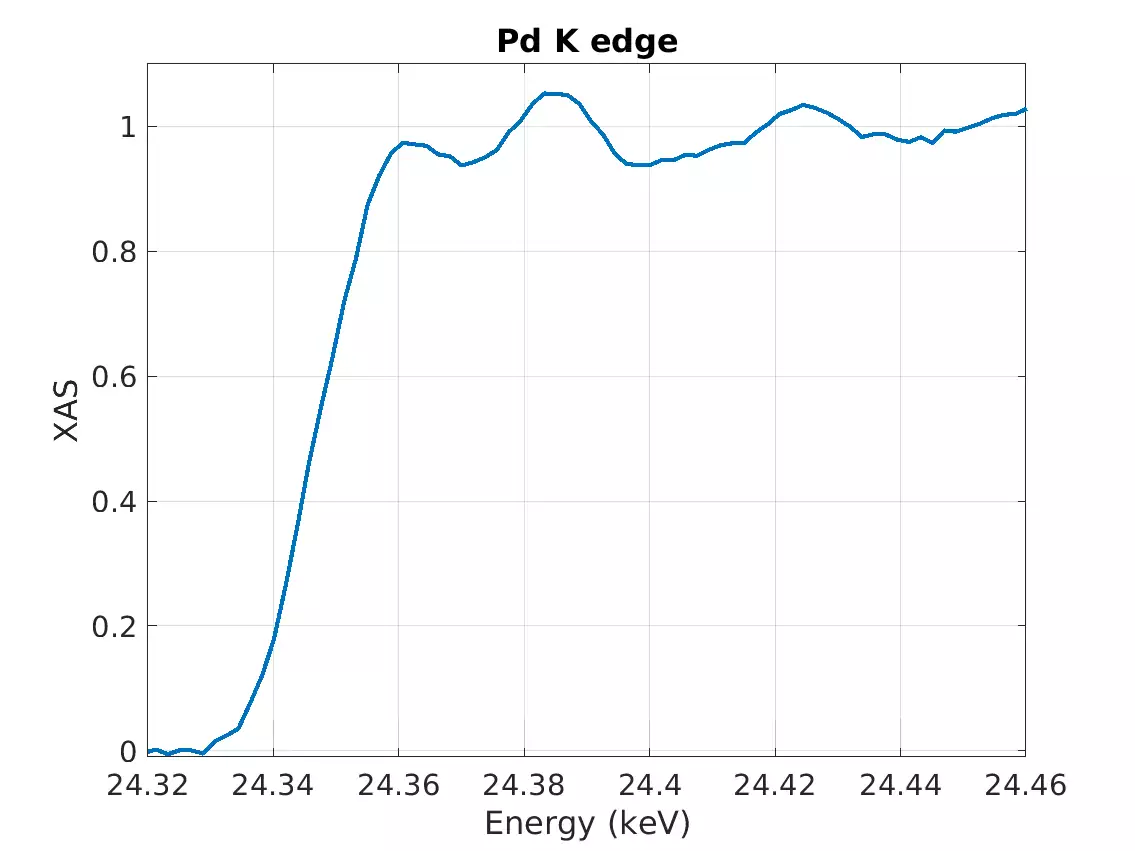
*The lowest-energy absorption edge we have measured has been Sb L1 edge (4.7 keV) and the highest-energy one has been the Pd K edge (24.4 keV).